Describe Atomic Structure Using the Terms Proton Neutron Electron
Excited state has extra energy and wants to combine isotopseion. Protons are positively charged but they are bonded.

Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science
A proton p has a relative mass of 1 and a positive 1 charge.
. Atomic Structure - describe the characteristics of protons neutrons and electrons in terms of location charge and mass. Ground is normal and lowest possible state of an. Protons are much heavier than electron Nucleon number and proton number 1 Proton number is the total number of protons in an atom.
B describe with the aid of diagrams the structure of an atom as containing protons and neutrons nucleons in the nucleus and electrons arranged in shells energy levels f deduce the numbers of protons neutrons and electrons in atoms and ions. Number of Electrons Number of Protons Atomic Number. An atom has two major parts in its structure.
A neutron n has a relative mass of 1 and has no charge. The nucleus center of the atom contains the protons positively charged and the neutrons no charge. This means that in any electrically neutral atom the number of protons in the nucleus often referred to as the nuclear charge is balanced by the same number of electrons outside the nucleus.
The outermost regions of the atom are called electron shells and contain the electrons negatively charged. - analyze the structure of the. Number of Protons Atomic Number 36.
Okay so a neutron isnt completely stable. Mainly there are three model preposed. P113 problems 69-79 all 69.
Neutrons are neutral iv. Number of Electrons Number of Protons Atomic Number 36. They are also known as Nucleons.
Most of the atoms weight is in the nucleus since electrons weigh hardly anything. Thomsons model of an atom Rutherford model. Or you can use the mass which is the sum of all neutrons and protons either in atomic mass units or daltons.
Describe a cathode ray tube and how it operates. It is sometimes represented by the symbol A so. Describe a cathode ray tube and how it operates.
This page describes the structure of atoms in terms of the three fundamental subatomic particles protons neutrons and electrons. Number of Neutrons Mass Number - Atomic Number 84 - 36 48. 2 Nucleon number is the total number of protons and neutrons in an atom.
Protons and neutrons are in the nucleus electrons are in the shells. Isotopes are defined with examples and nuclide notate eg. The periodic table is a chart of all the elements arranged in increasing atomic number.
Article by Hakimuddin Kuwakhedawala. A simple structure of a helium atom is given bel. Number of Neutrons Mass Number - Atomic Number.
In an atom the proton and neutron resides together inside the nucleus. Protons electrons and neutrons. There are some differences in detail but not in concept.
Hakimuddin Kuwakhedawala is based in India with over 15 years experience as a teacher. If we look again at the neutron structure both the down-quark and the electron occupy the. 3 Proton number is also known as atomic number while nucleon number is also known as mass number.
- illustrate the structure of the atom by using the Bohr model including the charge relative mass and location of the sub-atomic particles. Surrounding the nucleus are electrons negative charge in an electron cloud. A cathode ray tube or an CRT is a vacuum tube that contains one or more electron guns which generates the beams that accelerates it at a high velocity.
When describing an atom you use the atomic number number of protons. In shells around nucleus. Protons and neutrons-make up nucleus each 1 amu Electrons-make.
Protons and neutrons are arranged into nuclear shells similar to electron shells. The particles that contribute to the atoms mass protons and neutrons are contained within a very small central nucleus that has a diameter of about 1 10 15 m. The electrons are arranged in shells around the nucleus.
In which Z is the atomic number and N is the neutron number. A state the relative charges and approximate relative masses of a proton a neutron and an electron. 702 describe the structure of an atom in terms of protons neutrons and electrons and use symbols such as 146C to describe particular nuclei.
The principle quantum number n the orbital angular momentum l and the azimuthal angular momentum or magnetic quantum number m. The proton charge is exactly the same as the electron charge but of opposite sign. The number of electrons or protons is called the atomic number whereas the sum of protons and neutrons is called atomic mass.
Each element has a unique number of protons called the atomic number and when added to the number of neutrons forms a mass number. Atoms are made up of protons neutrons and electrons. Atoms contain protons neutrons and electrons.
Atoms have shell and a nucleus with its nucleus. - use atomic mass atomic number and charge to identify neutral atoms ions and isotopes. The mass number equals the sum of the numbers of protons and the number of neutrons in the nucleus.
4 In a neutral atom the total. An atom is composed of protons positive charge and neutrons neutral in an atomic nucleus. See also Atomic structure and radioactivity.
Objective - Today you will learn to describe and identify the characteristics of protons neutrons and electrons in terms of location chargeand mass- illustrate the structure of the atom by using the Bohr model including the charge relativemass and location of the sub-atomic particles- use atomic mass atomic number and charge to. Once again look at the atom. In addition to this it has three fundamental particles namely proton neutron and electron.
How would you describe the Structure of an Atom After the discovery of electron and proton the scientists started thinking of arranging these particles in an atom. Atoms consist of three basic particles. Top right picture is explained.
An electron e has a negligible about 000054 relative mass and a negative -1 charge. Protons are the carriers of positive electric charge in the nucleus. The atom is made of three fundamental particles.
They are the nucleus and the electron cloudThe nucleus is formed by protons and neutrons. The three dimensional state where an electron can be found is the orbital. 111 A Z N.
Thomson was the first scientist to propose a model for the structure of atom. Review Atomic Structure Electron Configuration Atomic Structure. The shells are characterized by 3 quantum numbers.
The electrons occupy the region around the nucleus. A Z N. Its well acknowledged that isolated neutrons will decay into a proton and electron the neutron having a half-life of 103 minutes.
Describe how protons neutrons and electrons relate to atomic structure. Number of Protons Atomic Number.
Atomic Structure Wghs Junior Science
The Structure Of The Atom Boundless Chemistry
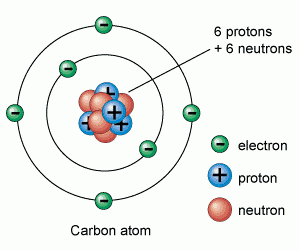
7 02 Describe The Structure Of An Atom In Terms Of Protons Neutrons And Electrons And Use Symbols Such As 146c To Describe Particular Nuclei Tutormyself Chemistry
No comments for "Describe Atomic Structure Using the Terms Proton Neutron Electron"
Post a Comment